Shenzhen Cell Valley won the bid for the procurement project of "GMP grade CAR T cell preparation" from Huazhong University of Science and Technology Union Shenzhen Hospital
On the afternoon of October 11, Shenzhen Cell Valley Biomedicine Co., Ltd. won the bid for the "GMP grade CAR T cell preparation" project in Huazhong University of Science and Technology Union Shenzhen Hospital, relying on excellent product quality, solid and efficient service style, top technical team and high-quality corporate reputation, with absolute advantages!
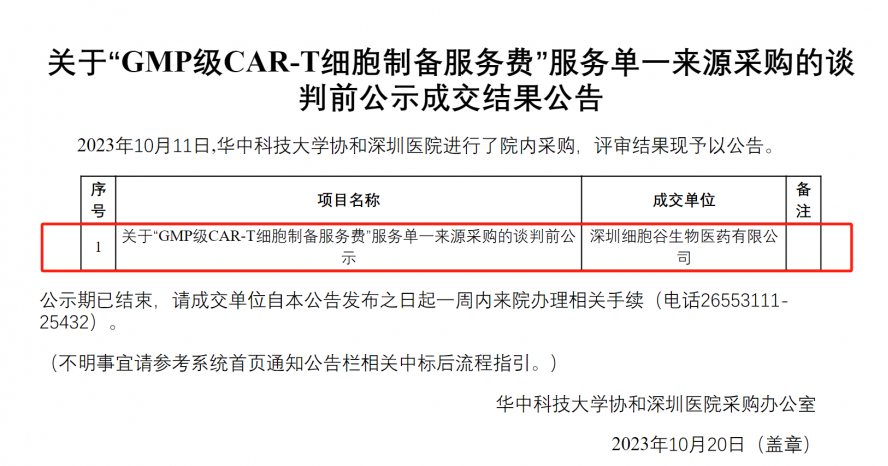
In the whole process of winning the bid, we have experienced several times of communication, negotiation and uation, from the initial determination of tender intention, to the submission of tender documents and quotations, and then to the fierce negotiations on the bidding site, witnessing the practical and meticulous service style of Shenzhen Cell Valley people.
Shenzhen Cell Valley Biomedicine Co., Ltd. won the bid, which means the official launch of the cooperation with Peking University Shenzhen Graduate School and Huazhong University of Science and Technology Shenzhen Union Hospital on the "CD19-CD22 dual-target CAR-T clinical research based on retroviral vector". Shenzhen Cell Valley is committed to enabling research and development, improving the speed of transformation, and reducing the cost of industrialization. Seek new life for patients, let good products out of the laboratory, truly R & D service clinical, and applied to clinical.
The winning bid is another milestone in the close cooperation between Shenzhen Cell Valley and the Hematology Department of Shenzhen Union Hospital of Huazhong University of Science and Technology, and the two sides will further cooperate in clinical research of CAR-T and other cell immunotherapy products in the future. Shenzhen Cell Valley provides GMP grade retroviral vectors and cell products with high safety, good quality and low price to ensure the safety and effectiveness of cell products, while focusing on the close integration of scientific research and clinical practice, and is committed to improving the therapeutic effect and promoting the overall development of cell and gene therapy industry.



